MDCisplatin
From HP-SEE Wiki
General Information
- Application's name: Molecular Design of Platinum Group Metal Complexes as Potential Non-classical Cisplatin Analogues
- Application's acronym: MDCisplatin
- Virtual Research Community: Computational Chemistry Applications
- Scientific contact: Nicolay Dodoff, dodoff@obzor.bio21.bas.bg
- Technical contact: Nicolay Dodoff, dodoff@obzor.bio21.bas.bg
- Developers: Acad. Roumen Tsanev Institute of Molecular Biology-BAS, Bulgaria
- Web site: Tobefilledin
Short Description
The search for novel metal-based carcinostatics with improved therapeutic characteristics with respect to the parent compound cisplatin remains a typical problem of biocoordination chemistry and anticancer drug design. The state-of-the-art in metalbased cytostatics becomes the design of non-conventional (non-classical) platinum complexes, differing considerably in their structure from cisplatin, displaying distinct mechanism of action, and, hence, quite promising for the treatment of cisplatin-resistant tumors. We have chosen the sulfonamides and thiosemicarbazones as suitable ligands forming platinum group metal complexes that are expected to give quite promising results in the light of the design of non-conventional metal-based cytostatics.
Problems Solved
Quantum chemical calculations at HF ab initio and DFT levels on platinum and other metal coordination compounds with expected or proved cytotoxic acivity. Geometry optimisation and estimation of conformational an spectroscopic properties.
Scientific and Social Impact
Elucidation of relationships between geometric and electronic structure, physicochemical properties and pharmacological activity of platinum an other metal coordination compounds.
Development of novel metal-based cytotoxic compound with potential significance for the chemotherapy of cancer.
Collaborations
- Coordination Chemistry Laboratory (CCL), Institute of General and Inorganic Chemistry - BAS
- Department of Chemistry, Aristotle University of Thessaloniki, Greece
Beneficiaries
Tobefilledin
Number of users
Tobefilledin
Development Plan
- Concept: Tobefilledin
- Start of alpha stage: Tobefilledin
- Start of beta stage: Tobefilledin
- Start of testing stage: Tobefilledin
- Start of deployment stage: Tobefilledin
- Start of production stage: Tobefilledin
Resource Requirements
- Number of cores required for a single run: Tobefilledin
- Minimum RAM/core required : 1 GB
- Storage space during a single run : 50 GB
- Long-term data storage : 200 GB
- Total core hours required: Tobefilledin
Technical Features and HP-SEE Implementation
- Primary programming language : FORTRAN/BASIC
- Parallel programming paradigm : MPI/OpenMP
- Main parallel code : GAUSSIAN, GAMESS
- Pre/post processing code : Own program
- Application tools and libraries : 256
Usage Example
In order to explore the capabilities of the free quantum-chemical software Firefly PCGamess, appropriate purely organic molecules were chosen, with the aid, on a later stage the program to be applied to metal-containing systems, like cisplatin analogues. The work done has thus an important methodological significance.
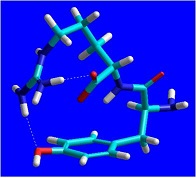
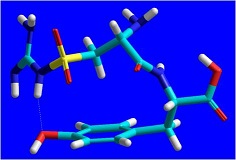
The endogenous peptides take part in the regulation of various adaptive reactions of the organism, including pain perception. The dipeptide kyotorphin (Tyr-Arg, Kyo) plays a role in pain modulation in the mammalian central nervous system (CNS), and is one of the most investigated neuropeptides. One of the successful strategies in the design of neuropeptides with enhanced stability and improved delivery to the CNS is that with the use of non-protein amino acids, like canavanive (Cav), a structural analogue and amtimetabolite of arginine (Arg). In our previous in vivo experiments we demonstrated that Tyr-Cav exerted a strong-reversible analgesic effect, more pronounced than that of Kyo. Bearing in mind these and the fact that norsulfoarginine (NsArg) is a structural analogue of arginine and canavanine, we synthesized a series of new peptides with expected analgesic activity, containing NsArg residues in their molecules: NsArg-Tyr, Tyr-NsArg, Tyr-NsArg-NH2 and Tyr-NsArg-OBzl. The conformational features of these dipeptides are of particular interest, both from theoretical and pharmacological point of view. Since no single-crystal X-ray diffraction data for the compounds are available until now, we undertook a quantum-chemical modelling of their structure. Here we present our preliminary computational results for Kyo (1) and NsArg-Tyr (2). Molecular mechanics (MM+ force field, HyperChem 7.5) conformational search for the two species was performed, and the global minimum-energy conformations thus obtained, were further optimized at HF ab initio (3-21G** basis set, Firefly PCGamess) level of theory. In both cases, specific, scorpion-like conformations are realized, with hydrogen bonds involving the guanidino-group and the phenolic hydroxyl.
An abstract for poster presentation has been prepared as follows: T. Pajpanova, T. Dzimbova, N. I. Dodoff, “Molecular Modelling of the Dipeptides Kyotorphin and Norsulfoarginine-tyrosine”, Fifty Years Institute of Molecular Biology Roumen Tsanev, Anniversary Molecualar Biology Conference, 2011, submitted.
Infrastructure Usage
- Home system: Tobefilledin
- Applied for access on: Tobefilledin
- Access granted on: Tobefilledin
- Achieved scalability: Tobefilledin cores
- Accessed production systems:
- Tobefilledin
- Applied for access on: Tobefilledin
- Access granted on: Tobefilledin
- Achieved scalability: Tobefilledin cores
- Tobefilledin
- Applied for access on: Tobefilledin
- Access granted on: Tobefilledin
- Achieved scalability: Tobefilledin cores
- Porting activities: Tobefilledin
- Scalability studies: Tobefilledin
Running on Several HP-SEE Centres
- Benchmarking activities and results: Tobefilledin
- Other issues: Tobefilledin
Achieved Results
Tobefilledin
Publications
- Tobefilledin
- Tobefilledin
Foreseen Activities
- Tobefilledin
- Tobefilledin